Junjun He
dMLLM-TTS: Self-Verified and Efficient Test-Time Scaling for Diffusion Multi-Modal Large Language Models
Dec 22, 2025



Abstract:Diffusion Multi-modal Large Language Models (dMLLMs) have recently emerged as a novel architecture unifying image generation and understanding. However, developing effective and efficient Test-Time Scaling (TTS) methods to unlock their full generative potential remains an underexplored challenge. To address this, we propose dMLLM-TTS, a novel framework operating on two complementary scaling axes: (1) trajectory exploration scaling to enhance the diversity of generated hypotheses, and (2) iterative refinement scaling for stable generation. Conventional TTS approaches typically perform linear search across these two dimensions, incurring substantial computational costs of O(NT) and requiring an external verifier for best-of-N selection. To overcome these limitations, we propose two innovations. First, we design an efficient hierarchical search algorithm with O(N+T) complexity that adaptively expands and prunes sampling trajectories. Second, we introduce a self-verified feedback mechanism that leverages the dMLLMs' intrinsic image understanding capabilities to assess text-image alignment, eliminating the need for external verifier. Extensive experiments on the GenEval benchmark across three representative dMLLMs (e.g., Lumina-DiMOO, MMaDA, Muddit) show that our framework substantially improves generation quality while achieving up to 6x greater efficiency than linear search. Project page: https://github.com/Alpha-VLLM/Lumina-DiMOO.
Probing Scientific General Intelligence of LLMs with Scientist-Aligned Workflows
Dec 18, 2025Abstract:Despite advances in scientific AI, a coherent framework for Scientific General Intelligence (SGI)-the ability to autonomously conceive, investigate, and reason across scientific domains-remains lacking. We present an operational SGI definition grounded in the Practical Inquiry Model (PIM: Deliberation, Conception, Action, Perception) and operationalize it via four scientist-aligned tasks: deep research, idea generation, dry/wet experiments, and experimental reasoning. SGI-Bench comprises over 1,000 expert-curated, cross-disciplinary samples inspired by Science's 125 Big Questions, enabling systematic evaluation of state-of-the-art LLMs. Results reveal gaps: low exact match (10--20%) in deep research despite step-level alignment; ideas lacking feasibility and detail; high code executability but low execution result accuracy in dry experiments; low sequence fidelity in wet protocols; and persistent multimodal comparative-reasoning challenges. We further introduce Test-Time Reinforcement Learning (TTRL), which optimizes retrieval-augmented novelty rewards at inference, enhancing hypothesis novelty without reference answer. Together, our PIM-grounded definition, workflow-centric benchmark, and empirical insights establish a foundation for AI systems that genuinely participate in scientific discovery.
MedQ-Bench: Evaluating and Exploring Medical Image Quality Assessment Abilities in MLLMs
Oct 02, 2025



Abstract:Medical Image Quality Assessment (IQA) serves as the first-mile safety gate for clinical AI, yet existing approaches remain constrained by scalar, score-based metrics and fail to reflect the descriptive, human-like reasoning process central to expert evaluation. To address this gap, we introduce MedQ-Bench, a comprehensive benchmark that establishes a perception-reasoning paradigm for language-based evaluation of medical image quality with Multi-modal Large Language Models (MLLMs). MedQ-Bench defines two complementary tasks: (1) MedQ-Perception, which probes low-level perceptual capability via human-curated questions on fundamental visual attributes; and (2) MedQ-Reasoning, encompassing both no-reference and comparison reasoning tasks, aligning model evaluation with human-like reasoning on image quality. The benchmark spans five imaging modalities and over forty quality attributes, totaling 2,600 perceptual queries and 708 reasoning assessments, covering diverse image sources including authentic clinical acquisitions, images with simulated degradations via physics-based reconstructions, and AI-generated images. To evaluate reasoning ability, we propose a multi-dimensional judging protocol that assesses model outputs along four complementary axes. We further conduct rigorous human-AI alignment validation by comparing LLM-based judgement with radiologists. Our evaluation of 14 state-of-the-art MLLMs demonstrates that models exhibit preliminary but unstable perceptual and reasoning skills, with insufficient accuracy for reliable clinical use. These findings highlight the need for targeted optimization of MLLMs in medical IQA. We hope that MedQ-Bench will catalyze further exploration and unlock the untapped potential of MLLMs for medical image quality evaluation.
TopoSculpt: Betti-Steered Topological Sculpting of 3D Fine-grained Tubular Shapes
Sep 04, 2025Abstract:Medical tubular anatomical structures are inherently three-dimensional conduits with lumens, enclosing walls, and complex branching topologies. Accurate reconstruction of their geometry and topology is crucial for applications such as bronchoscopic navigation and cerebral arterial connectivity assessment. Existing methods often rely on voxel-wise overlap measures, which fail to capture topological correctness and completeness. Although topology-aware losses and persistent homology constraints have shown promise, they are usually applied patch-wise and cannot guarantee global preservation or correct geometric errors at inference. To address these limitations, we propose a novel TopoSculpt, a framework for topological refinement of 3D fine-grained tubular structures. TopoSculpt (i) adopts a holistic whole-region modeling strategy to capture full spatial context, (ii) first introduces a Topological Integrity Betti (TIB) constraint that jointly enforces Betti number priors and global integrity, and (iii) employs a curriculum refinement scheme with persistent homology to progressively correct errors from coarse to fine scales. Extensive experiments on challenging pulmonary airway and Circle of Willis datasets demonstrate substantial improvements in both geometry and topology. For instance, $\beta_{0}$ errors are reduced from 69.00 to 3.40 on the airway dataset and from 1.65 to 0.30 on the CoW dataset, with Tree length detected and branch detected rates improving by nearly 10\%. These results highlight the effectiveness of TopoSculpt in correcting critical topological errors and advancing the high-fidelity modeling of complex 3D tubular anatomy. The project homepage is available at: https://github.com/Puzzled-Hui/TopoSculpt.
A Survey of Scientific Large Language Models: From Data Foundations to Agent Frontiers
Aug 28, 2025



Abstract:Scientific Large Language Models (Sci-LLMs) are transforming how knowledge is represented, integrated, and applied in scientific research, yet their progress is shaped by the complex nature of scientific data. This survey presents a comprehensive, data-centric synthesis that reframes the development of Sci-LLMs as a co-evolution between models and their underlying data substrate. We formulate a unified taxonomy of scientific data and a hierarchical model of scientific knowledge, emphasizing the multimodal, cross-scale, and domain-specific challenges that differentiate scientific corpora from general natural language processing datasets. We systematically review recent Sci-LLMs, from general-purpose foundations to specialized models across diverse scientific disciplines, alongside an extensive analysis of over 270 pre-/post-training datasets, showing why Sci-LLMs pose distinct demands -- heterogeneous, multi-scale, uncertainty-laden corpora that require representations preserving domain invariance and enabling cross-modal reasoning. On evaluation, we examine over 190 benchmark datasets and trace a shift from static exams toward process- and discovery-oriented assessments with advanced evaluation protocols. These data-centric analyses highlight persistent issues in scientific data development and discuss emerging solutions involving semi-automated annotation pipelines and expert validation. Finally, we outline a paradigm shift toward closed-loop systems where autonomous agents based on Sci-LLMs actively experiment, validate, and contribute to a living, evolving knowledge base. Collectively, this work provides a roadmap for building trustworthy, continually evolving artificial intelligence (AI) systems that function as a true partner in accelerating scientific discovery.
InternVL3.5: Advancing Open-Source Multimodal Models in Versatility, Reasoning, and Efficiency
Aug 25, 2025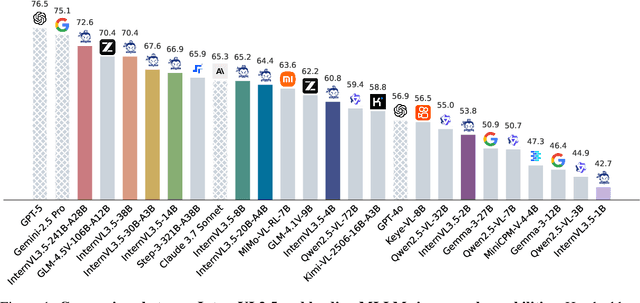
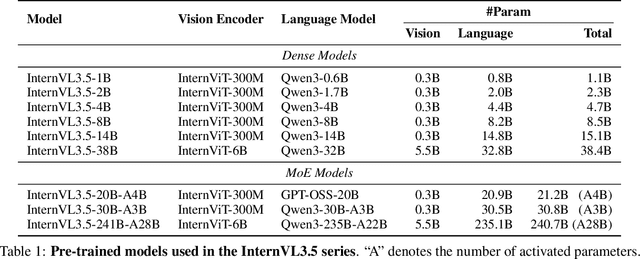
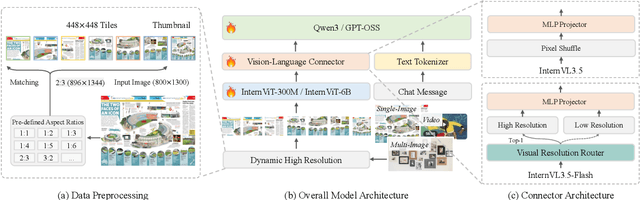
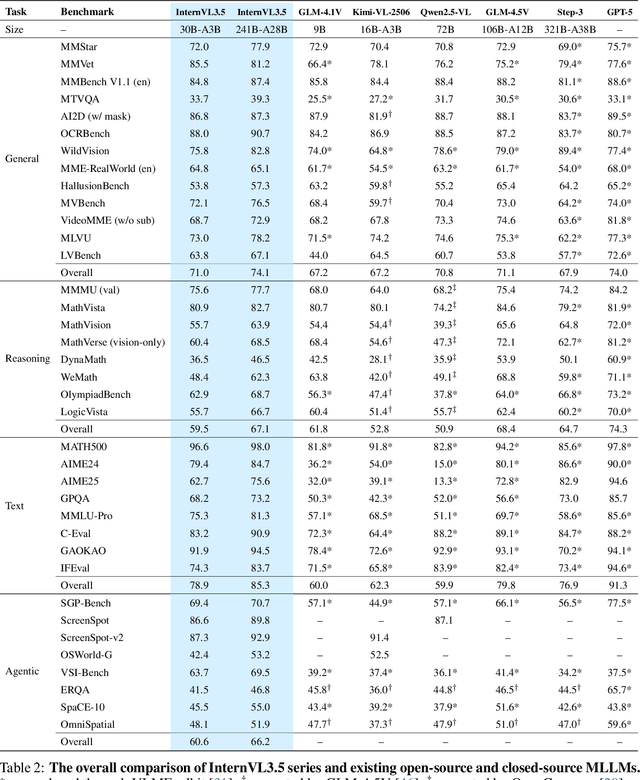
Abstract:We introduce InternVL 3.5, a new family of open-source multimodal models that significantly advances versatility, reasoning capability, and inference efficiency along the InternVL series. A key innovation is the Cascade Reinforcement Learning (Cascade RL) framework, which enhances reasoning through a two-stage process: offline RL for stable convergence and online RL for refined alignment. This coarse-to-fine training strategy leads to substantial improvements on downstream reasoning tasks, e.g., MMMU and MathVista. To optimize efficiency, we propose a Visual Resolution Router (ViR) that dynamically adjusts the resolution of visual tokens without compromising performance. Coupled with ViR, our Decoupled Vision-Language Deployment (DvD) strategy separates the vision encoder and language model across different GPUs, effectively balancing computational load. These contributions collectively enable InternVL3.5 to achieve up to a +16.0\% gain in overall reasoning performance and a 4.05$\times$ inference speedup compared to its predecessor, i.e., InternVL3. In addition, InternVL3.5 supports novel capabilities such as GUI interaction and embodied agency. Notably, our largest model, i.e., InternVL3.5-241B-A28B, attains state-of-the-art results among open-source MLLMs across general multimodal, reasoning, text, and agentic tasks -- narrowing the performance gap with leading commercial models like GPT-5. All models and code are publicly released.
EventRR: Event Referential Reasoning for Referring Video Object Segmentation
Aug 10, 2025Abstract:Referring Video Object Segmentation (RVOS) aims to segment out the object in a video referred by an expression. Current RVOS methods view referring expressions as unstructured sequences, neglecting their crucial semantic structure essential for referent reasoning. Besides, in contrast to image-referring expressions whose semantics focus only on object attributes and object-object relations, video-referring expressions also encompass event attributes and event-event temporal relations. This complexity challenges traditional structured reasoning image approaches. In this paper, we propose the Event Referential Reasoning (EventRR) framework. EventRR decouples RVOS into object summarization part and referent reasoning part. The summarization phase begins by summarizing each frame into a set of bottleneck tokens, which are then efficiently aggregated in the video-level summarization step to exchange the global cross-modal temporal context. For reasoning part, EventRR extracts semantic eventful structure of a video-referring expression into highly expressive Referential Event Graph (REG), which is a single-rooted directed acyclic graph. Guided by topological traversal of REG, we propose Temporal Concept-Role Reasoning (TCRR) to accumulate the referring score of each temporal query from REG leaf nodes to root node. Each reasoning step can be interpreted as a question-answer pair derived from the concept-role relations in REG. Extensive experiments across four widely recognized benchmark datasets, show that EventRR quantitatively and qualitatively outperforms state-of-the-art RVOS methods. Code is available at https://github.com/bio-mlhui/EventRR
S2-UniSeg: Fast Universal Agglomerative Pooling for Scalable Segment Anything without Supervision
Aug 09, 2025Abstract:Recent self-supervised image segmentation models have achieved promising performance on semantic segmentation and class-agnostic instance segmentation. However, their pretraining schedule is multi-stage, requiring a time-consuming pseudo-masks generation process between each training epoch. This time-consuming offline process not only makes it difficult to scale with training dataset size, but also leads to sub-optimal solutions due to its discontinuous optimization routine. To solve these, we first present a novel pseudo-mask algorithm, Fast Universal Agglomerative Pooling (UniAP). Each layer of UniAP can identify groups of similar nodes in parallel, allowing to generate both semantic-level and instance-level and multi-granular pseudo-masks within ens of milliseconds for one image. Based on the fast UniAP, we propose the Scalable Self-Supervised Universal Segmentation (S2-UniSeg), which employs a student and a momentum teacher for continuous pretraining. A novel segmentation-oriented pretext task, Query-wise Self-Distillation (QuerySD), is proposed to pretrain S2-UniSeg to learn the local-to-global correspondences. Under the same setting, S2-UniSeg outperforms the SOTA UnSAM model, achieving notable improvements of AP+6.9 on COCO, AR+11.1 on UVO, PixelAcc+4.5 on COCOStuff-27, RQ+8.0 on Cityscapes. After scaling up to a larger 2M-image subset of SA-1B, S2-UniSeg further achieves performance gains on all four benchmarks. Our code and pretrained models are available at https://github.com/bio-mlhui/S2-UniSeg
F^2TTA: Free-Form Test-Time Adaptation on Cross-Domain Medical Image Classification via Image-Level Disentangled Prompt Tuning
Jul 03, 2025Abstract:Test-Time Adaptation (TTA) has emerged as a promising solution for adapting a source model to unseen medical sites using unlabeled test data, due to the high cost of data annotation. Existing TTA methods consider scenarios where data from one or multiple domains arrives in complete domain units. However, in clinical practice, data usually arrives in domain fragments of arbitrary lengths and in random arrival orders, due to resource constraints and patient variability. This paper investigates a practical Free-Form Test-Time Adaptation (F$^{2}$TTA) task, where a source model is adapted to such free-form domain fragments, with shifts occurring between fragments unpredictably. In this setting, these shifts could distort the adaptation process. To address this problem, we propose a novel Image-level Disentangled Prompt Tuning (I-DiPT) framework. I-DiPT employs an image-invariant prompt to explore domain-invariant representations for mitigating the unpredictable shifts, and an image-specific prompt to adapt the source model to each test image from the incoming fragments. The prompts may suffer from insufficient knowledge representation since only one image is available for training. To overcome this limitation, we first introduce Uncertainty-oriented Masking (UoM), which encourages the prompts to extract sufficient information from the incoming image via masked consistency learning driven by the uncertainty of the source model representations. Then, we further propose a Parallel Graph Distillation (PGD) method that reuses knowledge from historical image-specific and image-invariant prompts through parallel graph networks. Experiments on breast cancer and glaucoma classification demonstrate the superiority of our method over existing TTA approaches in F$^{2}$TTA. Code is available at https://github.com/mar-cry/F2TTA.
ADAgent: LLM Agent for Alzheimer's Disease Analysis with Collaborative Coordinator
Jun 16, 2025Abstract:Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disease. Early and precise diagnosis of AD is crucial for timely intervention and treatment planning to alleviate the progressive neurodegeneration. However, most existing methods rely on single-modality data, which contrasts with the multifaceted approach used by medical experts. While some deep learning approaches process multi-modal data, they are limited to specific tasks with a small set of input modalities and cannot handle arbitrary combinations. This highlights the need for a system that can address diverse AD-related tasks, process multi-modal or missing input, and integrate multiple advanced methods for improved performance. In this paper, we propose ADAgent, the first specialized AI agent for AD analysis, built on a large language model (LLM) to address user queries and support decision-making. ADAgent integrates a reasoning engine, specialized medical tools, and a collaborative outcome coordinator to facilitate multi-modal diagnosis and prognosis tasks in AD. Extensive experiments demonstrate that ADAgent outperforms SOTA methods, achieving significant improvements in accuracy, including a 2.7% increase in multi-modal diagnosis, a 0.7% improvement in multi-modal prognosis, and enhancements in MRI and PET diagnosis tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge